MONOBLOC
Un implant monobloc conçu pour assurer une stabilité primaire ainsi qu’une ostéointégration optimales.
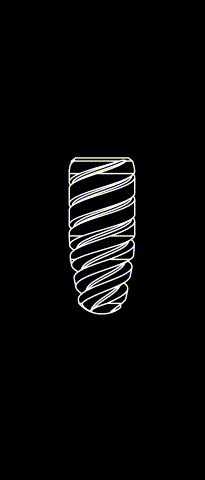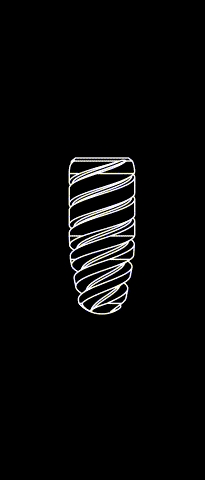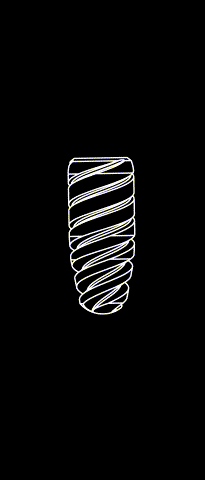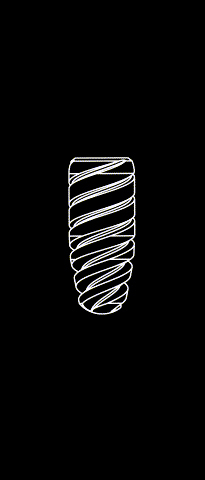
KONTACT™ MB
Optimisez l’espace biologique et préservez le complexe ostéo-muqueux
Discover a monoblock implant with a body design highly inspired by the iconic Kontact™ which benefits from a concave transgingival neck and an integrated universal conical abutment. The Kontact™ MB implant promotes and optimal bone and mucosa response.
Un implant monobloc novateur avec un héritage solide
Designed according to the BTLC ( Bone & Tissue Level Concept)
Combination of features and benefits from 1-piece and 2-pieces implants.
Monoblock implant to place the neck in juxtaposition or in subcrestal.
Protection of peri-implant soft tissues and bones due to its monoblock design and its smooth transgingival neck combined to a platform switching.
Pink anodised concave neck for a better aesthetic integration.
External connection with an integrated universal conical abutment (MUA – Multi-Unit Abutment), standard diameter for screw-retained prosthesis.
Performing design and implant surface
Cylindrical-conical design made of grade 4 Titanium (T60)
Sandbalsted-etched surface and SLA® hydrophilic
Excellent mechanical strength due to the absence of screwing holes in the neck and body of the implant
The monoblock concept with no implant-abutment junction will avoid disrupting the biological space when unscrewing and screwing during prosthetic phases.
Possibility of very narrow diameter:
L’absence de vis interne au corps de l’implant permet de réaliser un diamètre conçu pour les crêtes étroites afin de limiter les greffes osseuses.

CONSULTEZ LA DOCUMENTATION
Discover all benefits of the Kontact™ MB implant.

CONSULTEZ LE PROTOCOLE
Discover all the Kontact™ MB range and the included protocol.
Manufactured by: Biotech Dental. Biotech Dental – A simplified company limited by shares (S.A.S.) with a capital of 24,866,417 € – Corporate and Trade Register of Salon de Provence: 795 001 304 – SIRET: 795 001 304 00018 – TVA N°: FR 31 79 500 13 04. Class I, IIa and IIb medical devices for dental implantology. CE0459. Read the instructions carefully. Non contractual visuals. These medical devices are regulated health products that bear, under this regulation, the CE mark dedicated to health professionals.