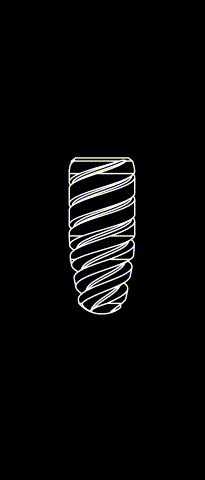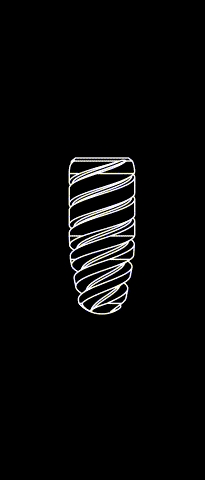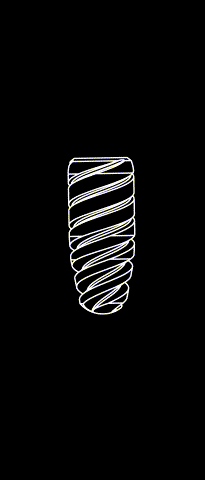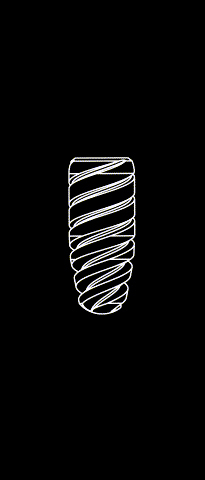
BONE LEVEL
The Kontact™ Bone Level range of dental implants is the result of a shared reflection between designers and engineers and is a concentrate of innovations for dental implantology. Thanks to its patented connectors and its wide range of prosthetic products, it meets all your indications and offers you great flexibility.

One surgical kit for all Kontact™ implants.
An ergonomic, simple kit to place all Kontact™ implants
KONTACT™
CHOOSE VERSATILITY, MAXIMIZE AESTHETIC RESULTS
Cylindrical-conical profile
Progressive bone condensation provides better primary stability.
Morse taper connection (10°)
Allows for a better bacterial seal and eliminates micromovements between the implant and the abutment. The mechanical strength of the implant-abutment pair is enhanced.
STSystem® indexing
Patented hexalobular indexing with six positions. Indexing with six lobes in the implant and three lobes on the abutment allows reliable, intuitive insertion of prosthetic components.
Surface Increaser
The secondary thread increases the developed surface and favours the distribution of forces on the immediate bone environment.
Constant Leaf
Cutting wings along the length of the implant optimise stability, reducing insertion force into the bone.
Chamfered and micro-structured implant neck
The advanced chamfer design allows for the retention of blood coagulum necessary for bone building, which strengthens the peri-implant tissues with additional surface area.
Atraumatic spherical apex
Protects the anatomical structure during surgery.
Implant available in narrow diameter ⌀3mm
A less invasive narrow Kontact™ implant, suitable for narrow interdental spaces as well as narrow ridges.

SEE THE DOCUMENTATION
Discover all the benefits of the Kontact™

CONSULT THE PROTOCOL
Discover the protocol of the Kontact™
Manufactured by: Biotech Dental.
Biotech Dental – simplified company limited by shares with a capital of €24,866,417 – trade and companies register of Salon de Provence, France: 795 001 304 – SIRET number [French business registration number]: 795 001 304 00018 – VAT No.: FR 31 79 500 13 04.
Class I, IIa and IIb medical devices for dental implantology. CE0459. Carefully read the instructions in the leaflet. Images for illustration purposes only. These medical devices are regulated health products that carry the CE mark. For health professionals only.
KONTACT™ XL
CHOOSE VERSATILITY, MAXIMIZE AESTHETIC RESULTS
Cylindrical-conical profile
Progressive bone condensation provides better primary stability.
Morse taper connection (10°)
Allows for a better bacterial seal and eliminates micromovements between the implant and the abutment. The mechanical strength of the implant-abutment pair is enhanced.
STSystem® indexing
Patented hexalobular indexing with six positions. Indexing with six lobes in the implant and three lobes on the abutment allows reliable, intuitive insertion of prosthetic components.
Surface Increaser
The secondary thread increases the developed surface and favours the distribution of forces on the immediate bone environment.
Constant Leaf
Cutting wings along the length of the implant optimise stability, reducing insertion force into the bone.
Chamfered and micro-structured implant neck
The advanced chamfer design allows for the retention of blood coagulum necessary for bone building, which strengthens the peri-implant tissues with additional surface area.
Atraumatic spherical apex
Protects the anatomical structure during surgery.

SEE THE DOCUMENTATION
Learn more about the finite element study of the Kontact™ XL.

SEE THE DOCUMENTATION
Learn more about the Kontact™ XL implant and the associated protocol.
Manufactured by: Biotech Dental.
Biotech Dental – simplified company limited by shares with a capital of €24,866,417 – trade and companies register of Salon de Provence, France: 795 001 304 – SIRET number [French business registration number]: 795 001 304 00018 – VAT No.: FR 31 79 500 13 04.
Class I, IIa and IIb medical devices for dental implantology. CE0459. Carefully read the instructions in the leaflet. Images for illustration purposes only. These medical devices are regulated health products that carry the CE mark. For health professionals only.
KONTACT™ N
Improve osseointegration and reduce treatment time
- accelerate osseointegration (via increased wettability),
- decrease the average level of marginal bone loss, thus favouring secondary stability of the implant
- early or immediate loading without compromising osseointegration
Cylindrical-conical profile
Progressive bone condensation provides better primary stability.
Highly hydrophilic nanostructured surface
In favour of osteoblast adhesion and proliferation.
Morse taper connection (10°)
Allows for a better bacterial seal and eliminates micromovements between the implant and the abutment. The mechanical strength of the implant-abutment pair is enhanced.
STSystem® indexing
Patented hexalobular indexing with six positions. Indexing with six lobes in the implant and three lobes on the abutment allows reliable, intuitive insertion of prosthetic components.
Surface Increaser
The secondary thread increases the developed surface and favours the distribution of forces on the immediate bone environment.
Constant Leaf
Cutting wings along the length of the implant optimise stability, reducing insertion force into the bone.
Chamfered and micro-structured implant neck
The advanced chamfer design allows the retention of blood coagulum that is not necessary for bone building, which strengthens the peri-implant tissues through increased surface area.
Atraumatic spherical apex
Protects the anatomical structure during surgery.
Implant available in narrow diameter ⌀3mm
A less invasive narrow Kontact™ implant, suitable for narrow interdental spaces as well as narrow ridges.
Manufactured by: Biotech Dental.
Biotech Dental – simplified company limited by shares with a capital of €24,866,417 – trade and companies register of Salon de Provence, France: 795 001 304 – SIRET number [French business registration number]: 795 001 304 00018 – VAT No.: FR 31 79 500 13 04.
Class I, IIa and IIb medical devices for dental implantology. CE0459. Carefully read the instructions in the leaflet. Images for illustration purposes only. These medical devices are regulated health products that carry the CE mark. For health professionals only.
KONTACT™ S
EFFICIENCY AND PERFORMANCE FOR OPTIMAL RESULTS
Morse taper connection (10°)
Allows for a better bacterial seal and eliminates micromovements between the implant and the abutment. The mechanical strength of the implant-abutment pair is enhanced.
STSystem® indexing
Patented hexalobular indexing with six positions. Indexing with six lobes in the implant and three lobes on the abutment allows reliable, intuitive insertion of prosthetic components.
Narrowed, chamfered and micro-structured implant neck
The narrowing of the neck reduces bone compression of the cortical bone. The micro-structured chamfer promotes the retention of blood coagulum which is necessary for bone reconstruction.
High performance surface finish
In pure grade 4 titanium (T60), their sandblasted and etched surface has been studied to optimise wettability and promote osseointegration.
Platform switching
Platform switching, in synergy with a stable and bacteria-proof morse taper connection, is an important factor in tissue stability. The aim is to limit the occurrence of peri-implantitis.
Atraumatic spherical apex
Protects the anatomical structure during surgery.
Implant available in narrow diameter ⌀3mm
A less invasive narrow Kontact™ implant, suitable for narrow interdental spaces as well as narrow ridges.

SEE THE DOCUMENTATION
Discover all benefits of the Kontact™ S implant.

CONSULT THE PROTOCOL
Learn more about the entire Kontact™ S implant range and associated protocol.
Manufactured by: Biotech Dental.
Biotech Dental – simplified company limited by shares with a capital of €24,866,417 – trade and companies register of Salon de Provence, France: 795 001 304 – SIRET number [French business registration number]: 795 001 304 00018 – VAT No.: FR 31 79 500 13 04.
Class I, IIa and IIb medical devices for dental implantology. CE0459. Carefully read the instructions in the leaflet. Images for illustration purposes only. These medical devices are regulated health products that carry the CE mark. For health professionals only.
KONTACT™ S+
EFFICIENCY AND PERFORMANCE FOR OPTIMAL RESULTS IN LOW DENSITY BONES
Morse taper connection (10°)
Allows for a better bacterial seal and eliminates micromovements between the implant and the abutment. The mechanical strength of the implant-abutment pair is enhanced.
STSystem® indexing
Patented hexalobular indexing with six positions. Indexing with six lobes in the implant and three lobes on the abutment allows reliable, intuitive insertion of prosthetic components.
Narrowed, chamfered and micro-structured implant neck
The narrowing of the neck reduces bone compression of the cortical bone. The micro-structured chamfer promotes the retention of blood coagulum which is necessary for bone reconstruction.
High performance surface finish
In pure grade 4 titanium (T60), their sandblasted and etched surface has been studied to optimise wettability and promote osseointegration.
Platform switching
Platform switching, in synergy with a stable and bacteria-proof morse taper connection, is an important factor in tissue stability. The aim is to limit the occurrence of peri-implantitis.
Atraumatic spherical apex
Protects the anatomical structure during surgery.

SEE THE DOCUMENTATION
Discover all benefits of the Kontact™ S+ implant.

CONSULT THE PROTOCOL
Learn more about the entire Kontact™ S+ implant range and associated protocol.
Manufactured by: Biotech Dental.
Biotech Dental – simplified company limited by shares with a capital of €24,866,417 – trade and companies register of Salon de Provence, France: 795 001 304 – SIRET number [French business registration number]: 795 001 304 00018 – VAT No.: FR 31 79 500 13 04.
Class I, IIa and IIb medical devices for dental implantology. CE0459. Carefully read the instructions in the leaflet. Images for illustration purposes only. These medical devices are regulated health products that carry the CE mark. For health professionals only.