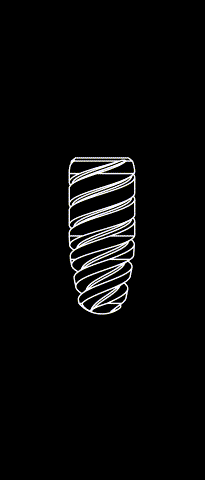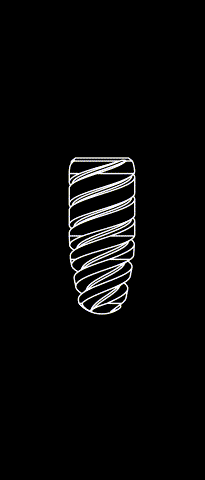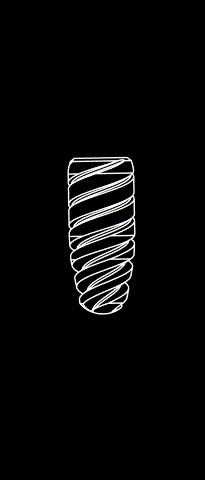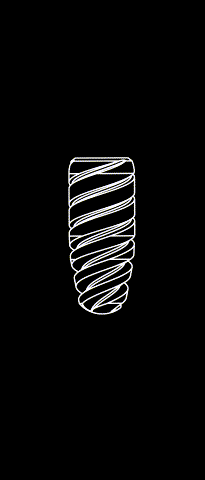
The subperiosteal implant
A design to suit your patient
Treat your complex cases in a single surgery!

1.
Unilateral posterior mandibular edentulism

2.
Complete mandibular edentulism

3.
Unilateral posterior maxillary edentulism

4.
Complete maxillary edentulism
Biomimetics, osseointegration and tissue integration
at the core of design
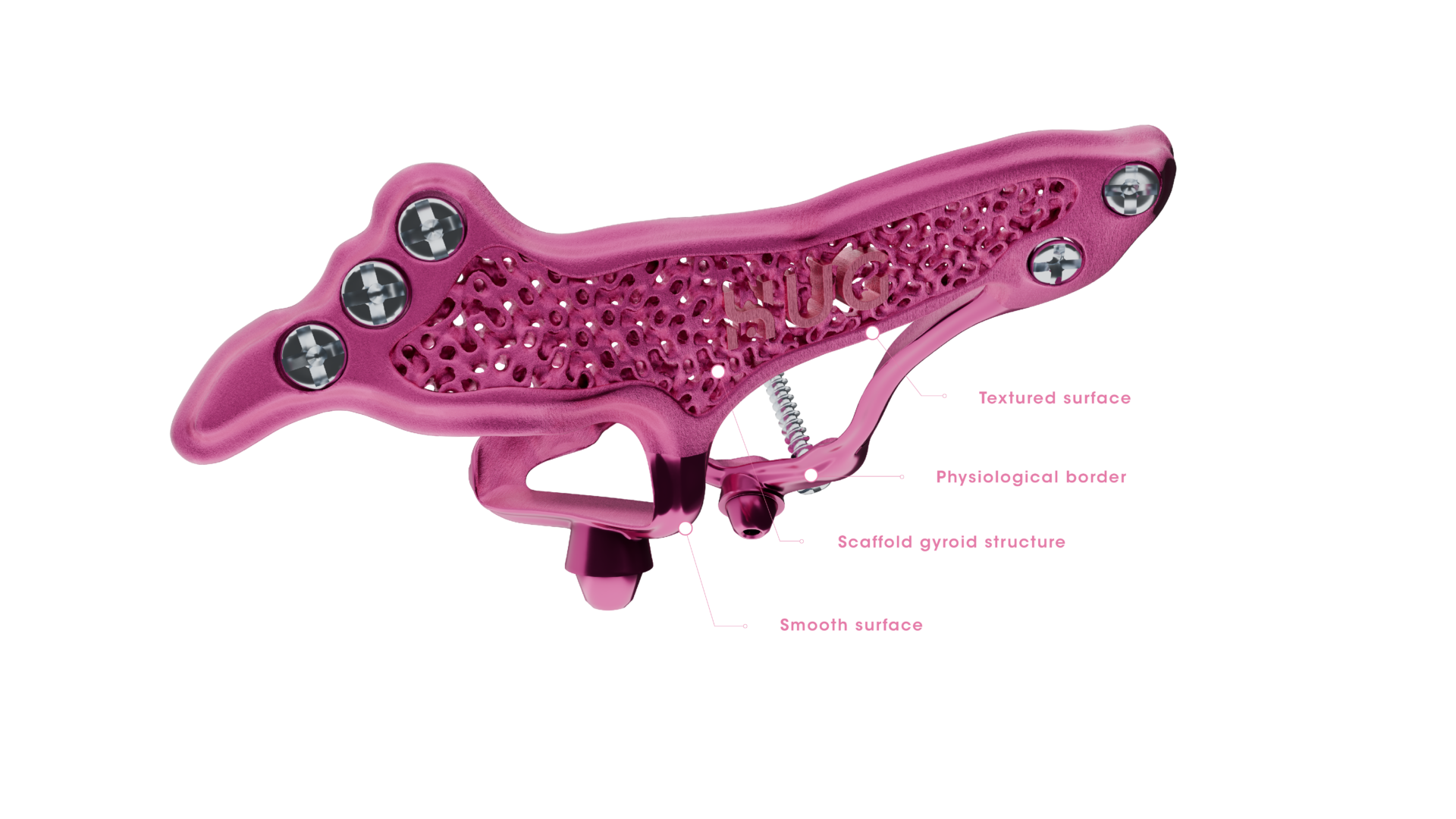
HUG prosthesis
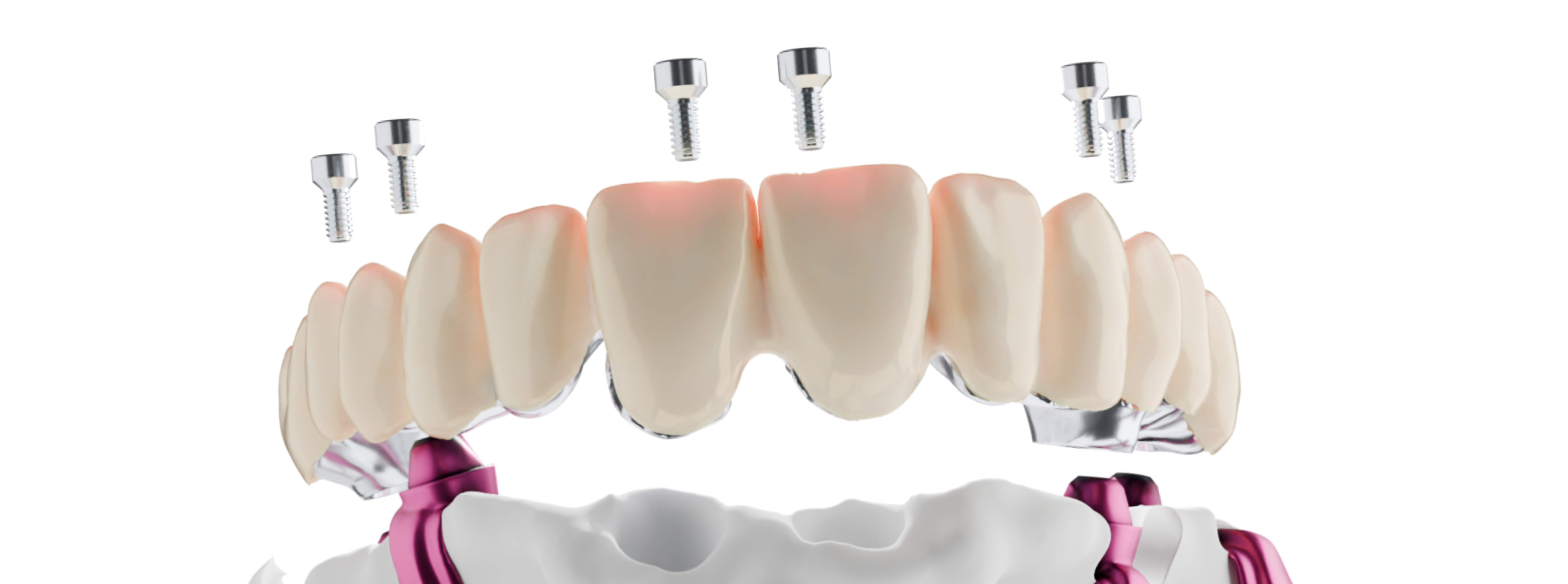
Part of the flow
In a single surgery, your patient leaves with a fixed prosthesis and titanium framework!

CONSULT THE BROCHURE
Hug® implant and Hug® cutting guide must be used by qualified and trained healthcare professionals – The design of Hug® implant and Hug® cutting guide is the responsibility of the prescriber. The target population concerns the intended use as well as the characteristics and performance claimed for this intended use – Class I and IIb custom-made device – Read the IFU instructions carefully. No refund from French social security – Manufactured by GLAD 3D , Innovation for medical devices – 246 rue des canesteu – 13300 Salon de provence – France – Tel.: +33(0)6 36 85 03 25 – Email: contact@gladmedical.com – www.glad-3d.com – A simplified company limited by shares (S.A.S.) with a capital of 1,000€ – Corporate and Trade Register of Salon de Provence: 918 599 176 – SIRET: 918 599 176 00016 – VAT No.: FR 89 91 859 91 76. Non contractual visuals.